What to Know About Pulse Oximeters
FRIDAY, Nov. 6, 2020 (HealthDay News) -- If you use an oxygen concentrator and a pulse oximeter at home, proper use is crucial, the U.S. Food and Drug Administration says.
Conditions such as asthma, lung cancer, chronic obstructive pulmonary disease, the flu and COVID-19 can all cause oxygen levels in the body to drop. When levels are too low, oxygen therapy may be required to boost them.
One way to get extra oxygen into the body is by using a prescription medical device called an oxygen concentrator.
But giving yourself too much or too little oxygen can be dangerous, so it's important to talk with your doctor and get a prescription before buying an oxygen concentrator for use at home, the FDA advises in a news release.
The FDA offers tips for safe oxygen concentrator use at home:
- Don't use it or any other oxygen product near an open flame or while smoking.
- Place the concentrator in an open space to reduce chances of device failure from overheating.
- Don't block any vents on the concentrator since it may impact device performance.
- Periodically check your device for any alarms to make sure you are getting enough oxygen.
- Don't make changes to the oxygen levels delivered by the device on your own. Consult your health provider.
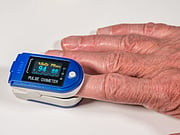
Oxygen levels are monitored by a small device called a pulse oximeter, which is placed on a finger, toe or forehead.
When using a pulse oximeter, the FDA says you should:
- Sit still and don't move the part of your body where the pulse oximeter is.
- Don't use the device on your hands when your hands are cold.
- Remove all fingernail polish if using the device on your hands.
Don't rely solely on the pulse oximeter. It's important to keep track of your symptoms and how you feel. Contact a doctor if your symptoms are serious or get worse.
If you are using a pulse oximeter to monitor your oxygen levels and are concerned about the reading, contact a health care provider, the FDA says.
More information
For more on oxygen therapy, go to the American Lung Association.
SOURCE: U.S. Food and Drug Administration, news release, November 2020

The news stories provided in Health News and our Health-E News Newsletter are a service of the nationally syndicated HealthDay® news and information company. Stories refer to national trends and breaking health news, and are not necessarily indicative of or always supported by our facility and providers. This information is provided for informational and educational purposes only, and is not intended to be a substitute for medical advice, diagnosis, or treatment.

